We explain what the atom is and what the story of its discovery was like. Also, what are its main characteristics.
What is the atom?
The atom is the smallest particle which can be divided matter .It is important to clarify that the definition of atom often uses the term "indivisible particle" to refer to the smallest particle that still retains the properties of the chemical element to which it belongs, but the atom is made up of even smaller particles (protons, neutrons , electrons), but they do not have the properties of the chemical element.
The first notions of the atom arose in the early nineteenth century , with the work of Dalton, who formulated the first atomic theory and first described the existence of spherical, tiny and indivisible particles that make up all matter and are equal to each other. in each chemical element.
Throughout that century and at the beginning of the next, the concept was refined, by scientists such as Thomson and Rutherford, until the formulation of the Böhr atomic model , proposed by Niels Böhr and according to which electrons revolve around of the nucleus at well-defined energy levels.
Atom characteristics
- It is a very small particle . The atom is the smallest particle that maintains the properties of the chemical element to which it belongs. It is the smallest unit into which matter can be divided; in fact, in Greek the word atom means "not divisible", although this term is not entirely correct, since the atom is made up of protons, neutrons and electrons.
- It is an infinitely light particle . The approximate mass of protons and neutrons is 1.6726 x 10 -27 kg and 1.6749 x 10 -27 kg respectively; that of electrons is even smaller: 9.1 x 10 -31 This makes atoms extremely light.
- Form molecules . Atoms group together to form molecules . Each type of molecule is the combination of a certain number of atoms linked together in a specific way, and a molecule can contain atoms of different chemical elements or of the same element.
- It is unchangeable . Each atom retains its structural characteristics, beyond being part of different molecules. During chemical reactions, atoms are neither created nor destroyed, but are organized differently by creating bonds between one atom and another.
- It has a fixed number of protons . What distinguishes chemical elements from each other (among many other characteristics) is the number of protons that their atoms have in the nucleus. The number of protons is represented by the letter Z and is called the "atomic number." The number of protons matches the number of electrons in an electrically neutral atom. The atomic number generally appears on the periodic table of the elements, located above the chemical symbol, on the left.
- It tends to instability . The normal tendency of the vast majority of atoms is to unite with other atoms (of the same type or different) to form stable groupings (that is, chemical compounds) because doing so leads to a situation of minimum energy and maximum stability. . By forming chemical bonds , they gain, lose, or share electrons. These junctions house energy that is eventually released as heat or light .
- It complies with the octet rule . What justifies the reactivity and formation of bonds is that the atoms comply with the Lewis octet rule, which indicates that the bonds respond to the need to acquire the electronic configuration that characterizes noble or "inert" gases, with eight electrons located in your last energy level. For example: the formation of the molecules of water and acetylene can be represented in the following diagram, where the electrons of carbon and oxygen are represented in red, while those of hydrogen are represented in black.
Structure of the atom
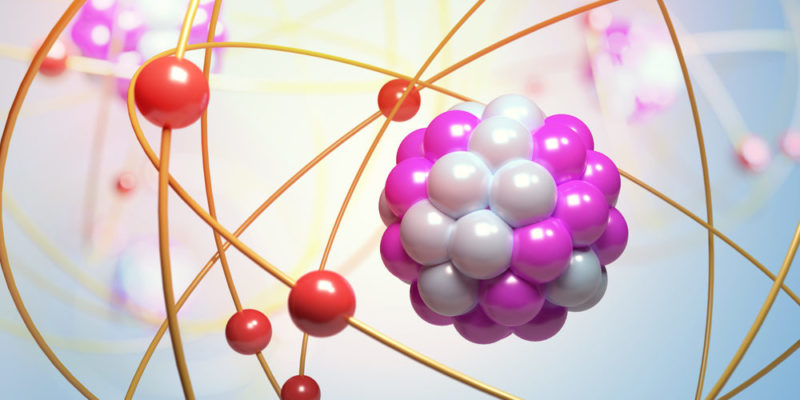
The atom is made up of a central nucleus and a cloud of electrons around that nucleus . The nucleus contains positively charged particles called protons and also uncharged particles known as neutrons. The negatively charged electron cloud is attracted to the protons in the nucleus by an electromagnetic force. In turn, electrons are characterized by atomic orbitals, which are mathematical functions that represent the probability of finding an electron in a region of space around the nucleus.
Atom mass
The mass of an atom is given mainly by the sum of the protons and the neutrons of its nucleus (since the mass of the electrons is infinitely small and therefore negligible). This parameter is called the mass number and is represented by the letter A.
Although the number of protons is identical for all atoms of a given chemical element , the number of neutrons can vary in some of those elements. This happens, for example, with carbon or nitrogen , which are elements that have several isotopes, such as carbon -14 or nitrogen -15.
The mass numbers (sum of protons and neutrons) of these two isotopes are 14 and 15, respectively . That is, carbon -14 has 6 protons and 8 neutrons, while nitrogen -15 has 7 protons and 8 neutrons.
Chemical reactivity
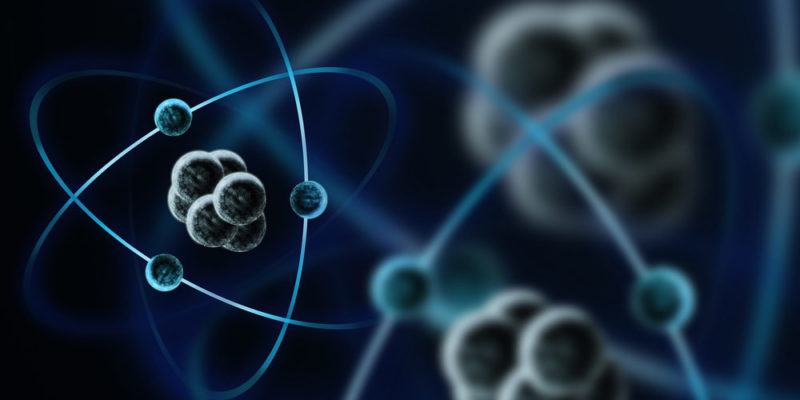
Although protons and neutrons are important in terms of mass and nuclear reactions, it is electrons (especially those at the last energy level of the electron cloud) that are responsible for the chemical reactivity of atoms.
It is the electrons that are ultimately going to allow infinite chemical compounds to be produced and decomposed all the time. That is, electrons are the subatomic particles that form the chemical bonds between atoms to form the various chemical compounds.
The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
Luke is passionate about fostering student involvement and connection. He studied psychology for his major and likes learning about the past. Luke aims to specialize in artificial intelligence and cybersecurity. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

