We explain what carbon dioxide is and what its general characteristics are. Also, its properties, solubility and more.
What is carbon dioxide?
Dioxide carbon is a gas that many chemical and biological processes is obtained as a final product . Its concentration in the atmosphere is rather low, although it has increased a lot in recent years.Carbon dioxide is made up of two oxygen atoms and one carbon atoms , so its chemical formula is CO 2 . This molecule has a linear and symmetric geometry, the Lewis structure that represents it is: O = C = O.
Structure and formula of carbon dioxide
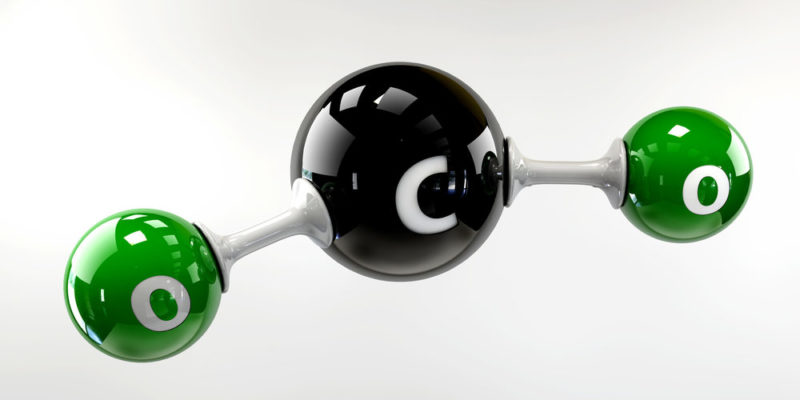 As its name implies, carbon dioxide is composed of two atoms of oxygen and one carbon atom , so the chemical formula CO 2 . The carbon dioxide molecule has linear and symmetric geometry. Formerly it was also called carbon dioxide or carbon dioxide.
As its name implies, carbon dioxide is composed of two atoms of oxygen and one carbon atom , so the chemical formula CO 2 . The carbon dioxide molecule has linear and symmetric geometry. Formerly it was also called carbon dioxide or carbon dioxide.
Carbon dioxide characteristics
- Physical properties . At room temperature and atmospheric pressure, carbon dioxide is a colorless and odorless gas, but it can solidify if subjected to temperatures below -79 ° C, as well as decompose if exposed to high temperatures (greater than 2000 ° C) .
- Solubility and density . Carbon dioxide is well soluble in water (each volume of water dissolves 0.9 volume of CO 2 ) and its density is 1.976 kg / m 3 .
- Cooling effect . Carbon dioxide in its solid form (which is achieved by exposure to low temperatures) forms dry ice. This is a very used and efficient refrigerant, which when losing cold does not transform into water (like common ice) but turns into gas (since at atmospheric pressure it is gaseous), which tends to minimize microbial contamination in fresh products. .
- Low reactivity . This gas is not combustible and, in general, it is not very reactive against other substances, making it ideal for use in fire extinguishers or home extinguishers. Being gaseous, it is directly compressed inside the extinguisher and does not require any additional discharge mechanism. In addition, it does not conduct electricity , and therefore it is used to put out fires in which there is a risk of electric shock.
- Acidity . Carbon dioxide dissolved in distilled water tends to bring the pH of distilled water to a slightly acidic value, because the formation of carbonic acid (H2CO3) occurs first and then the bicarbonate ion (HCO 3 - ).
How is carbon dioxide formed?
 Carbon dioxide is formed from various processes, the following stand out among them:
Carbon dioxide is formed from various processes, the following stand out among them:
- Combustion . The combustion of fossil and non-fossil materials, such as oil , coal, gas, produces carbon dioxide. For example: the combustion of methane (CH 4 ).
- Fermentation . The fermentation of sugars carried out by bacteria and yeasts , generally leads to the formation of acids and / or alcohols and CO 2 . For example: alcoholic fermentation.
- Breathing . The respiration of animals consists of taking in oxygen and releasing carbon dioxide. For example: cellular respiration.
- Reaction of carbonates with acids . Some salts , such as carbonates, can react with acids to form carbon dioxide. For example: reaction of calcium carbonate (CaCO 3 ) and hydrochloric acid (HCl).
What is carbon dioxide for?
The photosynthesis carried out by plants and some other photosynthetic organisms, such as algae , requires CO 2 , so that these beings consume a good part of the carbon dioxide in the Earth's atmosphere.Photosynthesis is a very important process as it tends to partially reverse the increasing accumulation of dioxide associated with the industrialization of urban centers .
Carbon dioxide and the greenhouse effect
 The greenhouse effect consists of the absorption of thermal radiation emitted by the earth's surface by certain gases . Then some of this thermal radiation is returned to the ground, heating the surface more than it should.
The greenhouse effect consists of the absorption of thermal radiation emitted by the earth's surface by certain gases . Then some of this thermal radiation is returned to the ground, heating the surface more than it should.The emission of gases, including carbon dioxide, has increased alarmingly in the last century . The concentration of CO 2 in the atmosphere is estimated to have risen by almost 100 ppm in the last 150 years.
This is related to the increase in the Earth's temperature and to a series of concomitant damages (melting of the polar ice caps, changes in natural vegetation, changes in animal species , etc.). Most of the carbon dioxide emissions come from industrial processes (mainly from the burning of fossil fuels) that take place in developed countries.
The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
Katheryn is a corporate attorney and finance specialist, conducting research daily to get you closer to financial security and freedom (even if you're just getting started). Her +600 articles published in Collaborative Research Group have already helped thousands of readers on the internet. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples
September 23, 2021

Dogs: Emergence, Features, Characteristics, Feeding and Breeds
September 24, 2021

Story: Definition, Elements, Structure, Features and Characteristics
September 24, 2021

Essay: Definition, Structure, Features, Characteristics, How to Do It
September 24, 2021
