We explain what a covalent bond is, its polarity and how it is classified. Also, what are its characteristics and some examples.
What is a covalent bond?
A covalent bond is a type of chemical bond in which two atoms are joined by sharing electrons in their most superficial atomic shell or their last atomic orbital (probability of finding an electron around the nucleus), and thus reaching the stable octet (according to the Gilbert Newton Lewis's "octet rule"). The atoms bound share one (or more) pairs of electrons.
This type of bond differs from the ionic bond in that the atoms that form it have an electronegativity difference of less than 1.7, while in the ionic bond they have a difference greater than 1.7.
Covalent bonds are formed between atoms of the same or different non-metallic elements and between a non-metal and hydrogen.
Covalent bonds are different from ionic bonds, since in the formation of ionic bonds there is a transfer of electrons from one atom to another, forming ions of opposite charges that are then electrostatically attracted. Furthermore, the ionic bond is formed between atoms of a metal and a nonmetal .
Octet rule
The "octet rule" was formulated by Gilbert Lewis at the beginning of the 20th century , to describe the tendency of atoms to join together to achieve a "complete" or "closed" outer electronic shell, according to their valence (number of electrons that you must accept or give up an atom to complete your last layer). This principle is the one that governs covalent bonds.
Said complete shell would consist of eight (8) electrons, so an atom with valence 6 would look for one more pair and an atom with valence 2 would seek to have up to six more, because in this configuration the atom enjoys a lot of stability, similar to that in they present the noble gases .
Differences with ionic bonding (electrovalent)
While the covalent bond, as has been said, consists of sharing pairs of electrons from the outer shell of the joined atoms, ionic bonds consist of transmitting or lending an electron between one atom and the other .
For this to occur, one of the two atoms must give up one or more electrons to the other atom. So the atoms must be of a metallic and a non-metallic element. Thus, positively charged ions are formed (cations, from metal) and negatively charged ions (anions, from non-metal) that are electrostatically attracted to form the bond.
Electric dipole
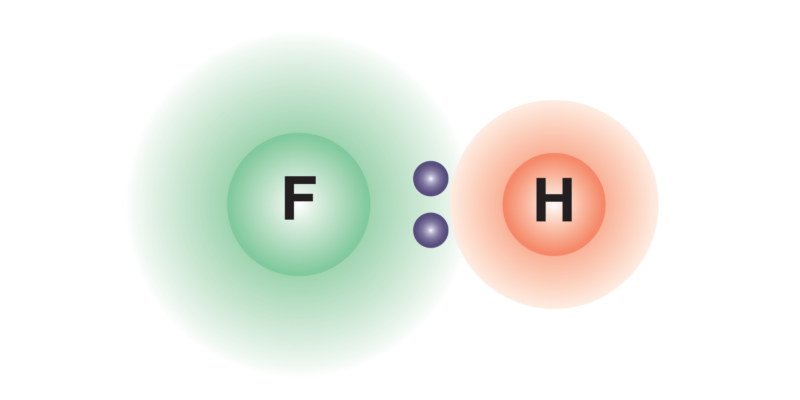
The covalent bonds between atoms of different chemical elements usually produce an uneven distribution of the electric charge density over the formed chemical compound, which generates an electric dipole (a system of two charges of opposite sign and equal magnitude). This occurs because one of the atoms is more electronegative than the other , so it will attract the electrons of the bond to itself with greater force, generating a negative charge density on it and a positive one on the other atom, which leads to the formation of the dipole.
This allows covalent molecules to join with similar ones and build more complex molecular structures.
In the event that the bond is formed with atoms of the same element, the distribution of charges on the chemical compound formed will be the same over its entire structure, so an electric dipole should not be produced.
Polarity
Polarity is a property of chemical compounds that have a non-uniform (uneven) distribution of charges over their structure, which is why it is closely related to the formation of electric dipoles . According to the presence or absence of polarity, it is possible to distinguish between polar covalent bonds (which form polar molecules ) and non-polar covalent bonds (which form non-polar or non-polar molecules).
- Polar covalent bonds . They occur between atoms of different chemical elements and have an electronegativity difference greater than 0.5. These bonds form the electromagnetic dipole.
- Nonpolar or nonpolar covalent bonds . They are formed between atoms of the same chemical element, whose electronegativity is the same. They are also formed between atoms of different chemical elements, but they have a very small electronegativity difference (less than 0.4). Since the electron cloud is attracted equally by both nuclei, the dipole is not formed in the molecule.
Covalent bond types
There are the following types of covalent bond:
- Simple . Atoms share a pair of electrons from their last shell (one electron from each). For example: HH, H-Cl.
- Double . Atoms contribute two electrons each, forming a four-electron double bond. For example: O = O, O = C = O.
- Triple . Atoms contribute three electrons to form three electronic pairs, that is, six electrons in total forming the triple bond. For example: N?N.
- Dative . A covalent bond in which one of the two atoms contributes two electrons and the other none. For example: NH 4 + .
Covalent bond breaking
When atoms are covalently bonded, they usually give off energy . Therefore, to break this bond it is necessary to supply that lost energy , which will vary according to the type of atoms bonded and the type of covalent bond formed. Thus, the binding energy is the total energy that is released when a mole of covalent bonds is formed and it is the same that would have to be applied to break that mole of bonds.
For example, to break the covalent bonds of 1 mole of hydrogen molecules (H 2 ) , it is necessary to apply 104 kilocalories / Kcal (435 kilojoules / kJ).
Types of covalent substances
Substances whose atoms have covalent bonds can be of two types:
- Molecular . They form molecules with low melting and boiling temperatures, thermally and electrically insulating, soft when they are solid and soluble in other substances of similar polarity (polar in polar and nonpolar in nonpolar). For example: oxygen (O 2 ).
- Reticular . They form crystalline networks of atoms (similar to ionic compounds) and that present high melting and boiling temperatures, hardness and solidity under normal conditions of pressure and temperature (1atm and 25 ° C), insolubility and are thermal and electrical insulators. For example: quartz.
Atomic Valencia
Valence is the number of electrons that an atom must give up or accept to complete its outermost shell . It is an essential piece of information when studying covalent bonds, as it tells us how many electrons the atom requires to achieve stability.
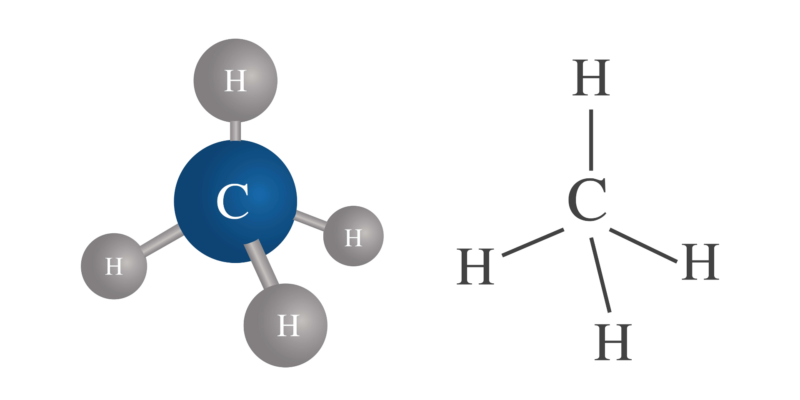
This valence can vary in the same atom . For example, carbon (C) has a valence of 4, hydrogen (H) has a valence of 1, but sulfur (S) can have a valence of 2, 4, and 6.
Examples of polar and nonpolar substances
According to the polarity of the covalent bonds that their molecules present, we can speak of polar compounds such as:
- Methanol, phenol, acetone, propionic acid.
- Ethane, Toluene, Isobutane, n-Pentane.
Examples of Covalently Bonded Compounds
- A molecule of oxygen (O 2 ): O = O (double bond)
- A molecule of hydrogen (H 2 ): HH (single bond)
- A molecule of carbon dioxide (CO 2 ): O = C = O (double bonds)
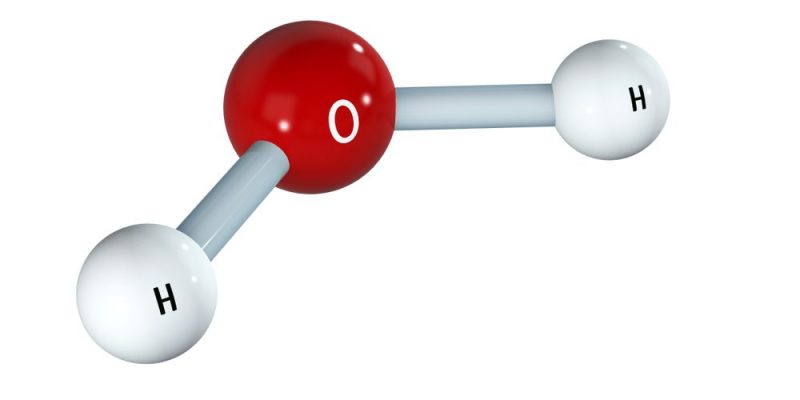
- A molecule of water (H 2 O): HOH (single bonds)
- A molecule of hydrochloric acid (HCl): H-Cl (single bonds)
- A molecule of nitrogen (N 2 ): N?N (triple bond)
- A molecule of hydrocyanic acid (HCN): HC?N (single bond and triple bond)
References:
The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
Anas is an editor of a prestigious publishing company in the United States. She studied Mathematics in Arizona. Anas is also a teacher and one of her long-term goals is to build an institution that offers free education to everyone who are financially not stable. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

