We explain what the Dalton atomic model is, what its main characteristics and the limitations it presents.
What is Dalton's atomic model?
Dalton's atomic model was the first conceptualization of the functioning, structure and arrangement of atoms . It was created between 1803 and 1807 by the English scientist John Dalton, who called it at the time "atomic theory" or "atomic postulates".
This theoretical elaboration allowed for the first time to provide a satisfactory explanation both to the Law of constant proportions –which establishes the fixed proportionality between reacting substances–, and to the Law of multiple proportions –which states that the proportions between substances that participate in a chemical reaction is always whole numbers. It also made it possible to explain the existence of numerous elemental substances from a finite set of constituent particles.
Dalton's atomic model is a relatively simple combinatorial model, which provided an explanation for almost all chemistry at the time and which laid the foundation for future developments and innovations in this and many other fields of science .
Dalton model postulates
- First postulate . The first postulate of Dalton's theory established that all matter is made up of elementary particles called “atoms” and that they cannot be divided, nor can they be destroyed. Nor, according to Dalton, cannot be created or changed in any chemical reaction.
- Second postulate . The atoms of any element are identical to each other, both in weight and in other characteristics; thus, all oxygen atoms are necessarily identical. Instead, atoms of different elements are distinguished from each other by their weight. From this postulate the notion of relative atomic weight arose, when comparing that of different atoms with that of hydrogen .
- Third postulate . Atoms cannot divide, regardless of whether they combine as a result of a chemical reaction. The combination of the same or different atoms will generate more complex compounds or substances, but always starting from the atom as the minimum fundamental unit of matter.
- Fourth postulate . The combination of the atoms of different elements can form different compounds depending on the amounts of these elements. For example, hydrogen peroxide (H 2 O 2 ) is made up of two oxygen atoms and two hydrogen atoms, while the water molecule is made up of one oxygen atom and two hydrogen atoms (H 2 O). That is, oxygen and hydrogen are present in these two chemical compounds, but in different proportions.
- Fifth postulate . Atoms of different elements can combine in amounts of two or more to form the different chemical compounds.
- Sixth postulate . Atoms cannot be created or destroyed during a chemical reaction.
- Seventh postulate . When atoms combine to form chemical compounds, they do so in a simple numerical ratio, never fractional.
Virtues of the Dalton model
 There are many achievements of the Dalton model, which marked the formal beginning of chemistry as we understand it today . Atomic theory provided a channel for the questions of chemistry in the 18th and 19th centuries, and laid the foundations for the subsequent explosion (in the 20th century) that would lead, hand in hand with technology , to new discoveries in the understanding of the matter. Dalton's theory was simple, effective, and advanced for its time.
There are many achievements of the Dalton model, which marked the formal beginning of chemistry as we understand it today . Atomic theory provided a channel for the questions of chemistry in the 18th and 19th centuries, and laid the foundations for the subsequent explosion (in the 20th century) that would lead, hand in hand with technology , to new discoveries in the understanding of the matter. Dalton's theory was simple, effective, and advanced for its time.
Limitations of the Dalton model
 It was impossible for Dalton to know in the 19th century what the 20th century would reveal in terms of understanding atoms, such as that they are made up of smaller, electrically charged matter : protons, neutrons, and electrons. In fact, advances in atomic energy have shown that the atom can indeed fission and fuse.
It was impossible for Dalton to know in the 19th century what the 20th century would reveal in terms of understanding atoms, such as that they are made up of smaller, electrically charged matter : protons, neutrons, and electrons. In fact, advances in atomic energy have shown that the atom can indeed fission and fuse.Another important limitation had to do with the lack of knowledge of atomic weights , as established by the periodic table (created later by Mandeleev and Meyer) and its periodic regularities and specific chemical properties. This limitation was due to the fact that Dalton believed that chemical compounds were formed with the least amount of elements possible, that is, that water was made up of an oxygen atom and a hydrogen atom (HO) and not by an oxygen atom and two hydrogen (H 2 O), as it really is. Nor could it account for the existence of isotopes.
Finally, Dalton thought that gases were necessarily composed of simple elements and the same type of atoms. This led him to contradict Gay-Lussac's results on volumetric relationships, which turned out to be proven true.
Predecessors of the Dalton model
There were similar postulates in antiquity, mainly those of the Greek philosophers Leucippus of Miletus (5th century BC) and his disciple Democritus (5th - 4th century BC), who preferred to think of the relationships of matter from the physical and not magic or divine will. However, his atomistic models were not based on scientific observation and experimentation but on logical reasoning.Another predecessor was the Irish Higgins , who proposed a similar but less successful theory in 1789.
Later models
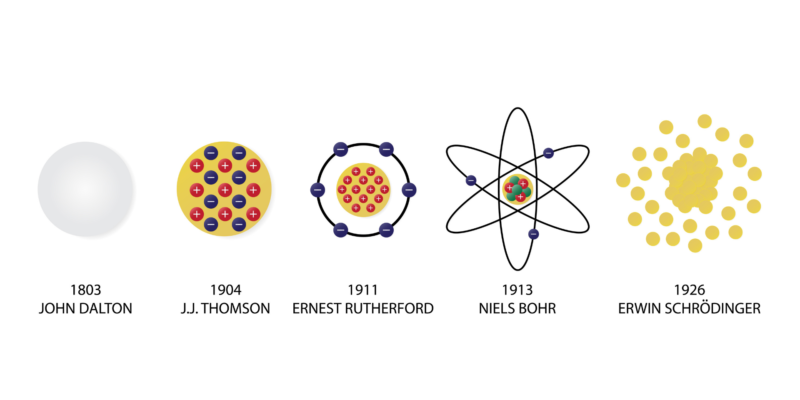
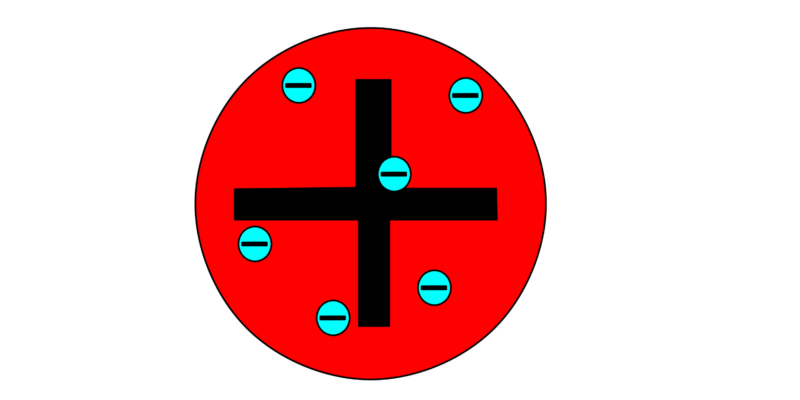 Other atomic models such as Thompson's (model known as "The Raisin Pudding") were made possible by Dalton's postulates, even though his indivisibility of the atom was contradicted by the discovery of the electron in the late 19th century.
Other atomic models such as Thompson's (model known as "The Raisin Pudding") were made possible by Dalton's postulates, even though his indivisibility of the atom was contradicted by the discovery of the electron in the late 19th century.In 1911, Rutherford proposed a model in which the atom is composed of a nucleus of positive charge where most of the mass of the atom is, around which electrons of negative charge orbit.
Then, in 1913, Niels Bohr released a model in which electrons orbit a positive nucleus, but only in certain allowed orbits.
These atomic models are followed by more modern models that are more specific and closer to the reality of the atom, but this has been possible thanks to technological advances.
The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
She has pursued her studies in The United States, where she has graduated in Business and Economics and is currently finishing her Master studies in International Economics and Finance. Miss. Amputee is fluent in three languages: English, Spanish and Russian and has elementary knowledge of French and Italian. She love exploring how Collaborative Research Group can become the best tool to achieve the (necessary) educational change. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

