We explain what gases are, how they are classified and the properties they present. Also, what are its characteristics and examples.
What are gases?
With the term gas we refer to one of the three main states of aggregation of matter (along with liquids and solids ). It is characterized by dispersion, fluidity and little attraction between its constituent particles.
Gases are the most volatile form of matter in nature and are extremely common in everyday life . Thus, when a substance is in a gaseous state we usually call it a gas.
Liquid or solid substances can be transformed into gas using different processes. This transformation implies a change in the physical properties of substances, such as their state of aggregation. However, their chemical properties do not change, since the substances continue to have the same chemical structure, that is, no breakdown of chemical bonds occurs nor are new substances generated.
Gases are found everywhere : from the heterogeneous mass of gases that we call the atmosphere and that we breathe as air , to the gases that are generated inside the intestine, the product of digestion and decomposition, to the flammable gases with which we feed our kitchens and ovens.
History of gases
The word gas was invented in the 17th century by the Flemish scientist Jan Baptista van Helmont , from the Latin term chaos ("chaos").
He chose the name for the apparent degree of disorder exhibited by the molecules of a gas. This state was also known as the "aeriform state" , but this term was deprecated.
The first laws on the behavior of gases were the consequence of their intensive study at the end of the same century, especially of their relationships between pressure, temperature and volume.
This led Émile Clapeyron to formulate the ideal law for all gases ("Ideal Gas Law") in 1834.
Ideal gas and real gas
An ideal gas is a model of gas created by the human being , and that does not have interactions between the particles that form it, that is, they do not have attraction or repulsion between them. On the other hand, a real gas does have these interactions.
The simpler the chemical formula of a real gas and the lower its reactivity, the more it can resemble an ideal gas. Thus, monatomic gases, for example helium (He), behave more similarly to ideal gases.
Gas laws
One of the laws most used to describe the behavior of gases is the Ideal Gas Law which, in turn, can be understood as the combination of other laws:
- Boyle-Mariotte law . It determines that the volume of a gas varies inversely proportional to the absolute pressure of the container where it is contained, if the temperature remains constant. It is expressed according to the equation:
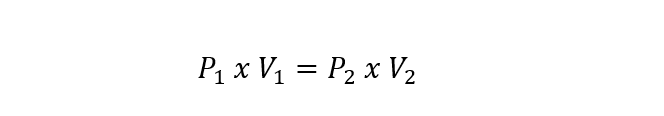
- Gay-Lussac Law . Explain that the pressure of a mass of gas whose volume is kept constant is directly proportional to its temperature (expressed in degrees Kelvin). This is represented as follows:
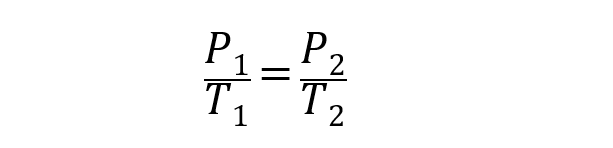
- Charles Law . It expresses that the temperature and the volume of a gas are directly proportional when the pressure is constant. This law is represented by the following equation:
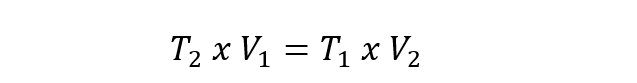
In all the above cases V 1 , P 1 and T 1 are the initial volume, pressure and temperature. While V 2 , P 2 and T 2 are the final volume, pressure and temperature.
- Avogadro's Law . It expresses that under the same conditions of pressure and temperature, volumes of different gaseous compounds contain the same number of particles.
- Ideal Gases Law . From the combination of the previous laws, the Ideal Gas Law is obtained, whose equation is represented as follows:
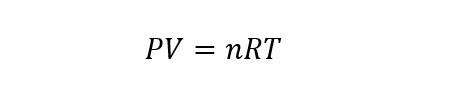
Where P , V, and T are pressure, volume, and temperature. While n is the number of moles of the gas and R is the ideal gas constant whose value is 8.31451 J / molK.
Types of gases
Gases can be classified according to their chemical nature in:
- Combustible or flammable . Those that can burn, that is, generate explosive or exothermic reactions in the presence of oxygen or other oxidants.
- Corrosive . Those that, when they come into contact with other substances, subject them to intense reduction or oxidation processes, causing damage to their surface or injuries if they are organic matter.
- Oxidizing . Those that allow a flame or a flammable reaction to be kept alive, since they induce combustion in other substances.
- Toxic . Those that represent a health hazard due to the reactions they introduce into the body of living beings , such as radioactive gases.
- Inert or noble . Those that show little or no reactivity, except in certain situations and conditions.
Properties of gases
 Gases have the following properties:
Gases have the following properties:
- They do not have their own volume . They occupy the volume of the container in which they are.
- They have no form of their own . They also assume that of their container.
- They can expand and contract . Like solids and liquids, gases expand if their temperature is increased, and they contract if they are cooled.
- They have great fluency . Gases flow much more than liquids because their particles have less interaction. They can easily move through a hole from one container to another.
- They have high diffusion . Gases can easily mix with each other due to the great movement of their particles.
- Solubility . Gases can be soluble in water or other liquids.
- They can be compressed . By applying pressure to a gas, its particles can be made closer together, that is, the gas is compressed.
Changes of states of gases

- Sublimation . It is a physical process of phase change, which allows a solid to be converted into a gas directly, without first going through a liquid stage. This process is rare and usually involves specific pressure and temperature conditions. We can observe it in dry ice (or ice) at room temperature: the solid block gives off a slight vapor, which is the substance recovering its original gaseous state.
- Boil . It is the process by which a liquid is transformed into a gas. It occurs when the entire mass of the liquid is heated to a temperature equal to its boiling point.
- Evaporation . It is an extremely common phase change process, which leads a liquid to turn into a gas when the temperature of the liquid is increased. It happens slowly and gradually. We put it into practice, for example, in the shower when very hot water turns into observable steam as a whitish cloud.
- Condensing . It is the opposite process to evaporation, that is, a phase change process that leads from the gaseous state to the liquid, due to the loss of heat energy. This lost energy causes the gas particles to vibrate more slowly, allowing them to approach and interact more closely, as occurs on cold glass on a rainy day, or on plants and other surfaces with dew.

- Reverse sublimation . It is the opposite path of sublimation, that is, the passage from the gaseous state to the solid state without first going through a moment of liquidity. This process requires very specific pressure and temperature conditions.
Plasma
The plasmatic state of matter is considered a fourth state of aggregation , but it has enormous similarities with the gaseous state, since it is basically an ionized gas, that is, a gas whose particles have lost electrons and have acquired a certain electromagnetic charge. . There are cold plasmas, as used in lamps "lava" or hot plasmas, such as fire surrounding the sun .
Examples of gases
 Some examples of gases are:
Some examples of gases are:
- Hydrogen (H 2 ) . It is the most common diatomic gas in the entire universe.
- Helium (He) . Tasteless, colorless and inert, it is the least soluble in water of all gases.
- Methane (CH 4 ) . It is a gaseous hydrocarbon with an unpleasant odor that is obtained as a product of the decomposition of organic matter.
- Air. It is the heterogeneous mixture of hydrogen, nitrogen , oxygen, argon and other gases that living beings breathe.
The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring to reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
MA student of the TransAtlantic Masters program at UNC-Chapel Hill. Political Science with a focus on European Studies. Expressed ideas are open to revision. He not only covers Technical articles but also has skills in the fields of SEO, graphics, web development and coding. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

