We explain what hydrocarbons are and how they are classified. Also, what are its characteristics and chemical and physical properties.
What are hydrocarbons?
Hydrocarbons are a group of organic compounds . Its molecules are made up of carbon and hydrogen atoms, organized in various structures depending on the type of hydrocarbon.
For the most part, hydrocarbons come from oil . This is because oil is the result of the decomposition of organic matter and therefore offers a large quantity and concentration of carbon and hydrogen .
Petroleum derivatives, that is, hydrocarbons, are involved in multiple industries , from aeronautics to the toy industry. Almost all the fuels used in transportation are derived from hydrocarbons, a use by which they create polluting waste ( carbon dioxide (CO 2 ) and carbon monoxide (CO)). For this reason, attempts are currently being made to replace them with other types of fuels and energy sources .
Hydrocarbons are a non-renewable resource , since they cannot be manufactured by humans .
Organic molecular structure of hydrocarbons
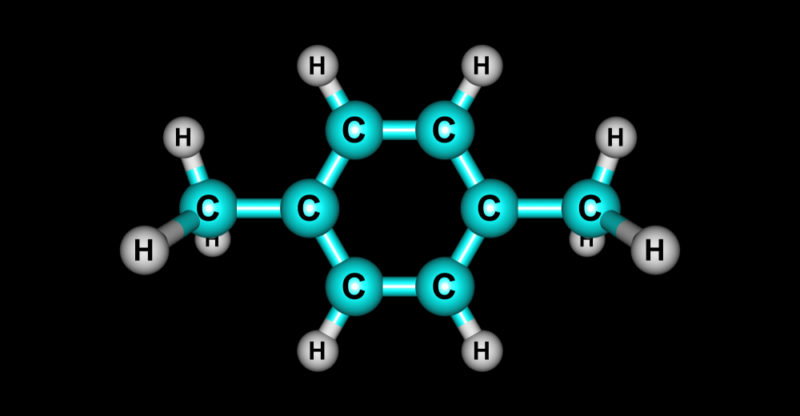
All substances whose molecules contain carbon atoms in their main structure that form bonds with other carbon atoms (carbon-carbon bond) and with hydrogen atoms (carbon-hydrogen bond) are considered organic . However, oxygen , sulfur , phosphorus and nitrogen can also be part of its structure . Hydrocarbons are part of organic molecules, which are only made up of carbon and hydrogen.
Although each hydrocarbon has a distinctive molecule , they all share a chain of carbon atoms in their molecular structure , each of which may also be linked to one or more hydrogen atoms.
Types of hydrocarbons
Aromatic hydrocarbons
Aromatic hydrocarbons have cyclic molecules, that is, the carbon atoms form a circle . Between the carbon atoms there are double bonds alternating with single bonds. One of the aromatic hydrocarbons is benzene, as well as all its derivatives.
Aliphatic hydrocarbons
Aliphatic hydrocarbons are those that do not form rings with double bonds alternated with single bonds, that is, they are not aromatic. The chains of the aliphatics can be open or closed.
- Open chain aliphatic hydrocarbons .
- Alkanes . The bonds between all carbon atoms are simple. The general formula is C n H 2n + 2 (n is the number of carbons).
- Alkenos . At least one of the bonds between the carbon atoms is double. The general formula is C n H 2n .
- Alkynes . At least one of the bonds between the carbon atoms is triple. The general formula is C n H 2n-2 .
- Closed chain aliphatic hydrocarbons . They are called cyclic and the chains of carbon atoms form rings, as in the case of aromatics. However, aliphatics do not have alternate with single double bonds. Many are called cycloalkanes.
Hydrocarbon saturation
It is said that a substance is saturated when in its molecule all the carbon atoms are joined to other atoms by simple bonds . The molecule is said to be saturated because single bonds cannot be broken and therefore no more hydrogen atoms can be added.The saturated hydrocarbons are alkanes and cycloalkanes . Unsaturated hydrocarbons are aromatics, alkenes, and alkynes.
Physical properties of hydrocarbons

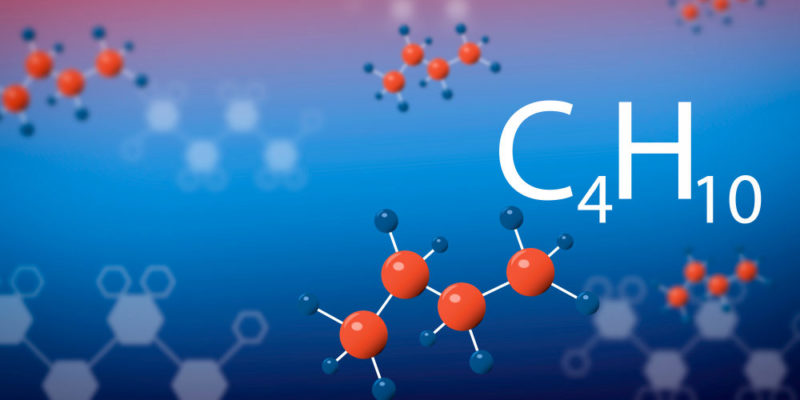
- Its boiling point increases as the size of the hydrocarbon increases (the number of carbon atoms), although the molecular geometry of these molecules also influences. This is because the intermolecular forces are greater when the molecule is larger. For example, the boiling point of butane (C 4 H 10 ) is 0 ° C while the boiling point of nonane (C 9 H 20 ) is 150.8 ° C.
- Its density also increases when the molecule is larger.
- They are insoluble in water . This is because they are apolar substances, that is, the densities of electrical charges in the different regions of each molecule cancel out. On the other hand, water is a polar molecule, and in chemistry the phrase is often used: similar dissolves similar.
Chemical properties of hydrocarbons
- Combustion . Hydrocarbons can go to complete or incomplete combustion (oxidation). They begin to oxidize in the presence of oxygen or in the presence of a heat source. One of the substances resulting from complete combustion is carbon dioxide (CO 2 ) and from incomplete combustion, carbon monoxide (CO). That is why hydrocarbons are polluting substances when used as fuel.
- Pyrolysis . It is the decomposition of organic compounds when they are heated to high temperatures without the presence of oxygen. For example, when alkanes are exposed to a temperature of 800 ° C, they can decompose to form alkenes, smaller alkanes, and free hydrogen.
- Halogenation . Under the presence of light , especially ultraviolet rays, alkanes react together with halogens , and produce halogenated derivatives.
Uses of hydrocarbons

Hydrocarbons are used mainly as fuel for transport and industry , but also in electric generators. In addition, they are the raw material for lubricants and greases for vehicles, as well as asphalt.
Hydrocarbons are processed to make all kinds of plastics , acrylics, nylon, gloves, paints , synthetic fibers, containers, adhesives, insecticides, detergents, refrigerants, and fertilizers.
Hydrocarbon degradation

Hydrocarbons are pollutants not only due to the residues produced when they ignite, but also when oil (a mixture of hydrocarbons) is spilled on the land or in the water. Despite being organic substances, hydrocarbons are not usually biodegradable .
However, research has been developed to address this problem . For this, a combination of bacteria is used that degrade the hydrocarbon molecules in a chain, that is, a bacterium manages to break the molecule to make it "edible" for another bacterium.
Nomenclature of hydrocarbons
To name hydrocarbons , a series of prefixes (at the beginning of the name) and suffixes (at the end of the name) are used to indicate the number of bonds and atoms.
Examples of prefixes according to the number of carbon atoms: Met (one carbon atom), Et (two), Prop (three), But (four), Pent (five), Hex (six), Hept (seven), Oct (eight), Non (nine).
Suffixes according to the type of hydrocarbons
- Alkanes : -ano. For example: butane (alkane with four carbon atoms).
- Alkenes : -no. For example: pentene (alkene with five carbon atoms).
- Alkynes : ino. For example: ethyne (alkyne with two carbon atoms).
In the case of hydrocarbons that have double or triple bonds, a number that corresponds to the position of the first carbon that participates in such multiple bonds must be entered as a prefix to the suffix -eno (if it is alkene) and to the suffix -ino (if it is alkene). alkyne).
Examples of Hydrocarbons
- Methane . CH 4 (alkane)
- Ethane . CH 3 - CH 3 (alkane)
- Propane . CH 3 - CH 2 - CH 3 (alkane)
- Butane . CH 3 - CH 2 - CH 2 - CH 3 (alkane)
- But-1-ino or 1-butyne . CH ? C - CH 2 - CH 3 (alkyne)
- Hep-3-ino or 3-heptyne . CH 3 - CH 2 - C ? C - CH 2 - CH 3 (alkyne)
MA student of the TransAtlantic Masters program at UNC-Chapel Hill. Political Science with a focus on European Studies. Expressed ideas are open to revision. He not only covers Technical articles but also has skills in the fields of SEO, graphics, web development and coding. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

