We explain what hydrochloric acid is, what its formula is like and the properties it has. In addition, its characteristics and harmful effects.
What is hydrochloric acid?
Hydrochloric acid, muriatic acid , salt spirit , marine acid , etching , salfumán and even hydrochloric acid is a binary chemical compound whose molecules are made up of a chlorine atom and a hydrogen atom , so its chemical formula is HCl.
It is an aqueous solution of hydrogen chloride gas (of identical chemical composition), highly irritating and corrosive.
This compound, hydrogen chloride, can be produced artificially or it can also be generated naturally, as a consequence of volcanic eruption, the burning of hydrocarbons or even by certain living beings .
To obtain hydrochloric acid, it is enough to make the hydrogen chloride come into contact with the water .
Importance of hydrochloric acid
Hydrochloric acid plays important roles in the human chemical industry , as it enables the production of more complex substances.
It also allows the elimination of residues in a container where a chemical reaction has occurred, which facilitates the generation of another reaction in that same container without involving any component generated in the previous reaction.
On the other hand, the fact that our own bodies secrete it means that it is a useful substance in controlled quantities , but that it should in no way be ingested or inhaled.
Hydrochloric acid properties

Chemical properties
- Reacts with many metals to form hydrogen. It also reacts with water, releasing heat. May react with strong oxidizing agents.
- It is used as a reagent to generate many chemical reactions. It is quite corrosive.
- It can dissolve metals (forming oxidized metal ions), organic tissues , or even salts and minerals.
- Its pH is less than 1, that is, it is extremely acidic .
- Its appearance is a transparent or yellowish liquid , depending on the concentration of hydrogen chloride, which is a slightly yellow gas, non-flammable and heavier than air .
- Although very dangerous, compared to other common strong acids in chemistry, it is relatively less dangerous to handle.
- Its physical properties (such as specific pH, density, and its melting and boiling points) vary according to its concentration in water. For example, the boiling point for a 10% hydrochloric acid aqueous solution is 103 ° C, while for one with 38% it is 48 ° C.
Hydrochloric acid in the human body
 Hydrochloric acid is a very powerful irritant , capable of damaging any organic tissue with which it comes in contact. Exposure to this substance as a gas (hydrogen chloride) can irritate the respiratory tract and, depending on its concentration, interruption of the respiratory cycle and death by suffocation.
Hydrochloric acid is a very powerful irritant , capable of damaging any organic tissue with which it comes in contact. Exposure to this substance as a gas (hydrogen chloride) can irritate the respiratory tract and, depending on its concentration, interruption of the respiratory cycle and death by suffocation.In the aqueous solution (hydrochloric acid), it is capable of producing irritation skin or corrode absolutely producing chemical burns and possible disfigurement or death. In addition, its mixture with bleach and other oxidizing agents produces the highly toxic dichloro gas.
On the other hand, the so-called gastric juices , whose task is to break down the chewed food and allow its absorption through the intestines, occurs thanks to the presence of approximately 3% of hydrochloric acid in our stomach.
This acid allows protein denaturation and does not damage a healthy stomach, thanks to the resistance of the internal layers of this organ, and the sodium bicarbonate secreted by some cells (and other organs such as the pancreas) to regulate the level of acidity.
In some cases, however, this process can get out of control and lead to heartburn , ulcers, or worse disease.
Hydrochloric acid uses
 Hydrochloric acid is a cheap, strong and volatile acid with many applications. It is used, for example:
Hydrochloric acid is a cheap, strong and volatile acid with many applications. It is used, for example:
- In the manufacture of cleaners, solvents, and other cleaning chemicals.
- To remove limestone (calcium carbonate) scale from other minerals.
- To regulate the acidity of other chemical solutions.
- To dissolve the mineral part of the bones, in the manufacture of gelatin.
- To dissolve the oxide layers of metals in the metallurgical industry.
- In the synthesis of other chemical materials such as iron trichloride (FeCl 3 ) or the synthesis of organic chlorides.
How was hydrochloric acid discovered?
 Its discovery is often attributed to Jabir ibn Hayyan (Geber), wrongly. This compound was known in the Middle Ages by alchemists , who called it the spirit of salt or acidum salis .
Its discovery is often attributed to Jabir ibn Hayyan (Geber), wrongly. This compound was known in the Middle Ages by alchemists , who called it the spirit of salt or acidum salis .The first experiments to obtain hydrogen chloride took place in the 17th century in Germany , by Johann Rudolf Glauber, and in 1772 in England by Joseph Priestley, who obtained it in a significant percentage of purity .
Its composition of hydrogen and chlorine was demonstrated that same year by the also English Humphry Davy.
How do you get hydrochloric acid?
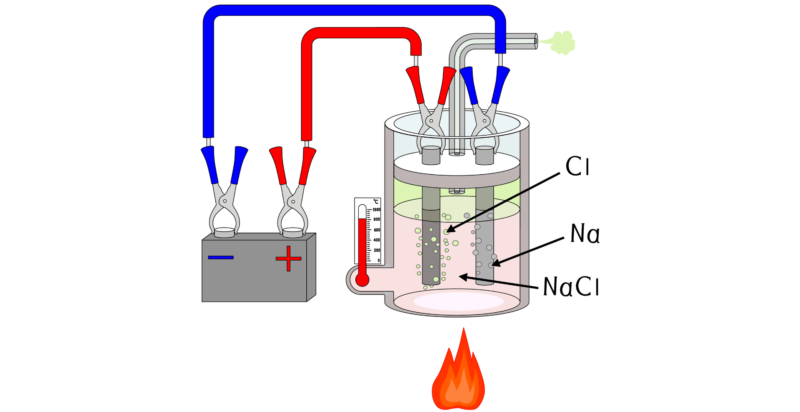 In laboratories, hydrochloric acid is obtained by adding sulfuric acid (H 2 SO 4 ) to common salt (NaCl) , heating the mixture to 150 ° C.
In laboratories, hydrochloric acid is obtained by adding sulfuric acid (H 2 SO 4 ) to common salt (NaCl) , heating the mixture to 150 ° C.On a large scale, another method is used, generally consisting of electrolysis of a common salt solution to produce dichloro (Cl 2 ) , sodium hydroxide (NaCl) and dihydrogen (H 2 ). Then the dichloro combines with dihydrogen, both gases, forming HCl. This is an exothermic reaction.
What is a chemical burn?
A chemical burn is a harmful and painful reaction that occurs when living tissue is exposed to a corrosive substance (very acidic or very alkaline).It is a substance that deteriorates organic matter and causes the substances that compose it to denature and dissolves it into simpler substances.
These burns can present different symptoms depending on the compound that causes them but, in general, they are immediate and extremely painful. In addition, they occur without an external source of heat (although in many cases they are due to an exothermic chemical reaction) and can cause deformities, loss of body tissue or death.
The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
Luke is passionate about fostering student involvement and connection. He studied psychology for his major and likes learning about the past. Luke aims to specialize in artificial intelligence and cybersecurity. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

