We explain what acetic acid is, what its formula is like and the properties it presents. In addition, its characteristics, uses and examples.
What is acetic acid?
Acetic acid, methylcarboxylic acid or also ethanoic acid is an organic compound that can be found as an acetate or ethanoate ion ([C 2 H 3 O 2 ] - ) . It is present in vinegar, a liquid that gives it its characteristic sour smell and taste.
It is a weak acid , a common result in certain fermentation processes , such as those that take place in wine or fruit. It is widely used in organic chemistry applications and in various industrial processes.
Its massive production in the world is due to the fact that it is a useful compound in obtaining other organic substances , such as acrylic acid.
Acetic acid formula
Acetic acid has the chemical formula C 2 H 4 O 2 and the semi-developed formula CH 3 COOH. From there it follows that it is a methyl group (CH3-) linked by a single bond from a carbon atom to a carboxyl group (-COOH).
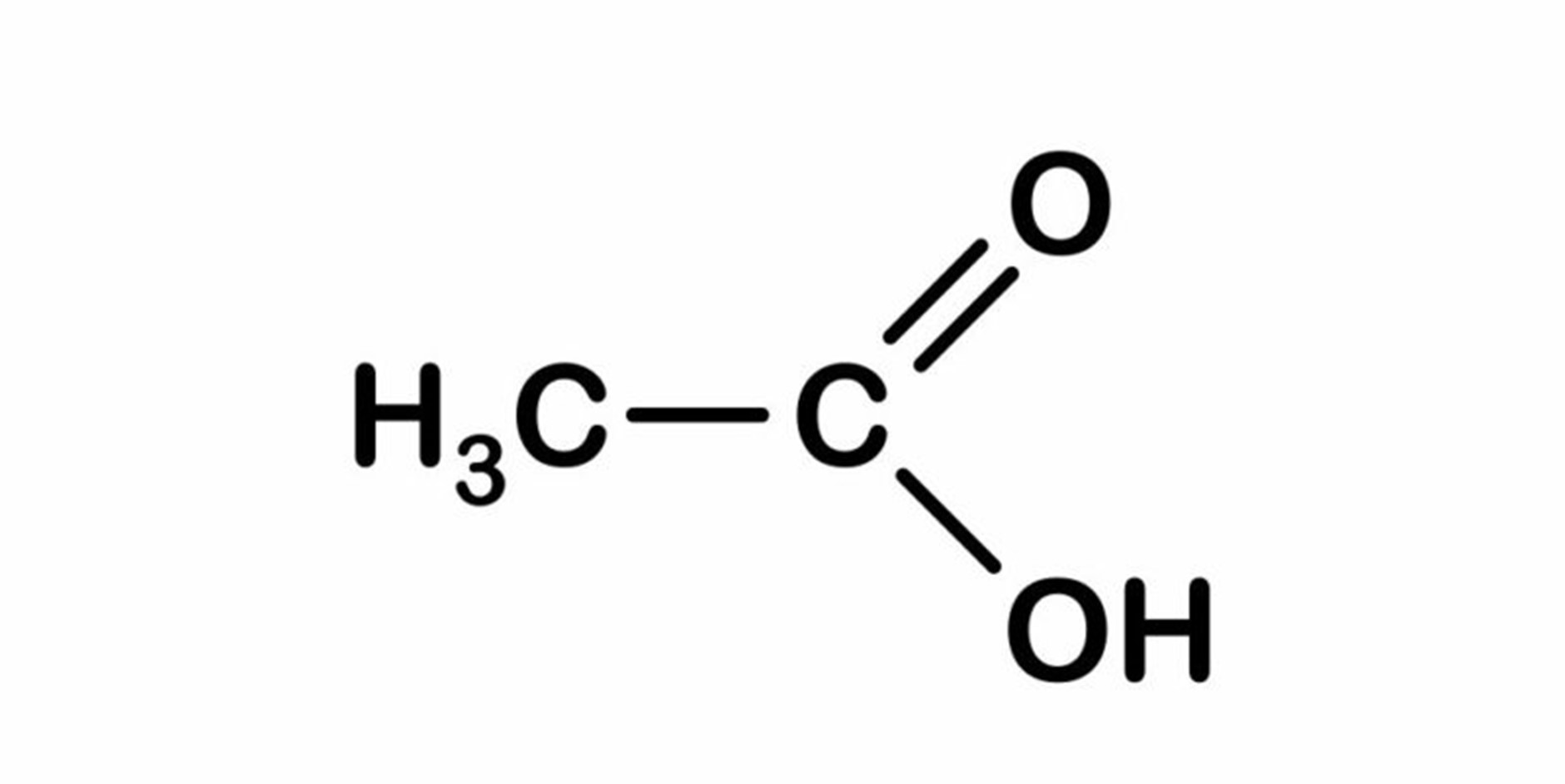
Acetic acid solubility

Acetic acid is quite a polar substance because it has a polar group (-COOH), although it also has a certain apolar character due to its group (-CH3). Due to the presence of both groups in its structure, it is a substance that is soluble in water (polar solvent) , but it is also soluble in many less polar organic substances.
Acetic acid molecular weight
- Molecular weight . Acetic acid has a molecular weight of 60.05 g / mol.
- Density . The density of acetic acid is 1049 kg / m3.
- Appearance . It can be a clear liquid with a strong vinegar odor and acid taste, or a colorless solid in the form of small regular crystals.
Vinegar and acetic acid
 Vinegar contains 3% to 5% acetic acid dissolved in water , along with small amounts of tartaric acid (C 4 H 6 O 6 ) and citric acid (C 6 H 8 O 7 ).
Vinegar contains 3% to 5% acetic acid dissolved in water , along with small amounts of tartaric acid (C 4 H 6 O 6 ) and citric acid (C 6 H 8 O 7 ).It was discovered for the first time in winemaking since the poorly guarded barrels in which air and oxygen were strained , underwent the fermentation process of the bacterium Mycoderma aceti , which converts ethyl alcohol into vinegar. It was said then that the wine was "chopped".
Vinegar, however, is an important part of human gastronomy and is produced from different sources: apples, grapes or artificial alcohols.
It is essential in the preparation of pickles, ceviche, pickles, or as a dressing for salads. It is also used in the preservation of food as it slows down the decomposition processes of organic matter.
Acetic acid properties
Acetic acid is a fairly weak acid , despite being flammable and corrosive at the same time, as well as a hygroscopic substance (which absorbs water from humidity in the environment).In high concentrations it can, therefore, irritate the skin, eyes or mucous membranes , both by inhalation and swallowing. It is an acid often secreted by living beings , as a metabolite and substrate for acetyltransferase enzymes .
Its boiling point is 118 ° C, while its melting point is 17 ° C.
Glacial acetic acid
 Glacial acetic acid is called the result of the freezing of acetic acid , in which the water that is part of the compound forms easily removable crystals of the compound. Once the water crystals have been separated from the acetic acid crystals, the acid is returned to a liquid state.
Glacial acetic acid is called the result of the freezing of acetic acid , in which the water that is part of the compound forms easily removable crystals of the compound. Once the water crystals have been separated from the acetic acid crystals, the acid is returned to a liquid state.Thus, when we speak of glacial acetic acid, we refer to anhydrous acetic acid , that is, without water.
Danger of acetic acid
Generally speaking, the acetic acid that you come in contact with in vinegar and other solutions is in such low concentrations that it is not hazardous at all.However, in high concentrations, it is an irritating acid capable of causing skin burns , permanent eye damage , and mucosal irritation.
It is not a particularly flammable compound , but in solutions greater than 25%, its gases can be just as corrosive and dangerous to human and animal life , which is why it is handled in a fume hood.
Obtaining acetic acid
Some of the main ways to obtain acetic acid are through the following reactions:
- Carbonylation of methanol . Reaction between methanol and carbon monoxide resulting in acetic acid.
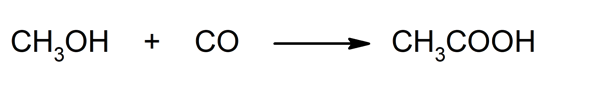
- Ethylene oxidation . Ethylene is oxidized using a metal as a catalyst, palladium (Pd) in this case.

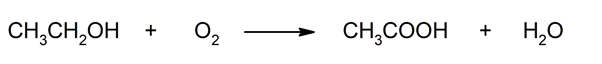
- Oxidative fermentation . In the presence of oxygen, bacteria of the genus Acetobacter can produce acetic acid from certain alcoholic compounds.
Acetic acid applications
 Acetic acid has numerous applications in different human industries, such as:
Acetic acid has numerous applications in different human industries, such as:
- It is used as an additive in the control of wax moths (a disease called galleriosis) in beekeeping.
- It is an important component (forming salts or esters) to make nylon, rayon, cellophane and other synthetic films.
- It is used in fixing substances for the preservation of organic tissues in laboratories (such as formaldehyde).
- It is used in chemicals for photographic development.
- It is used as a dye to reveal lesions the Virus of the Human Papillomavirus (HPV) in medicine.
- It is a common component of commercial cleaners and stain removers.
- It has many culinary uses as vinegar: to wash vegetables and greens, as a dressing, etc.
The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
MA student of the TransAtlantic Masters program at UNC-Chapel Hill. Political Science with a focus on European Studies. Expressed ideas are open to revision. He not only covers Technical articles but also has skills in the fields of SEO, graphics, web development and coding. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

