We explain what aggregation states are and how they are classified. Also, what are their characteristics and the changes they present?
What are states of aggregation?
States of aggregation of matter (or simply states of matter ) are called the phases or moments that the different known substances present , according to the existing union forces between the particles that compose them. Four phases of matter are traditionally known: liquid, solid, gas and plasma.
Each of these phases or moments, called "states", has its own physical characteristics , different from those that the same substance presents in other different states, despite the fact that its chemical constitution (the atomic composition of the particles) does not change at all. that compose them).
It is understood, then, that all matter is in some type of phase at a given moment , but can be taken to another by varying the temperature and/or pressure to which it is subjected, thus giving rise to a series of physical processes called “phase change methods”: solidification, melting, sublimation, deposition, condensation, vaporization, ionization, and deionization.
Types of states of matter

Four types of states of matter are known: solid, liquid, gaseous and plasmatic , each one different in variables such as hardness, resistance, malleability, fluidity, volume and cohesion, as well as in the relationship between its determined particles.
However, it is possible to bring matter to states of aggregation that do not ordinarily occur in nature but rather in very specific and controlled laboratory conditions: Bose-Einstein condensate, Fermi condensate, supersolid or quark-gluon plasma, the latter still hypothetical in nature.
Solid state

The solid state is recognizable because matter has a defined body, with its own volume and constant shape , depending on the substance in question. Its atoms are forming narrow and rigid structures, which offers resistance to external forces.
In general, they are resistant to fragmentation , have little or no fluidity, high cohesion and a shape memory that gives them elasticity, that is, the ability to recover their shape if they are removed from their original configuration.
Examples of matter in a solid state are ice, stone, ceramics , wood , bone .
Liquid state

Matter in a liquid state has a much looser union between its atoms than in the case of solids, which gives it fluidity, its main characteristic.
This means that matter does not have a certain form but assumes that of the container in which it is contained.
In general, liquid matter has less cohesion , kinetic energy movement, fluidity, diffusion, little compressibility and contraction in the presence of cold (except water).
Examples of matter in a liquid state are water , mercury , blood , milk .
Gaseous state

Matter in a gaseous state is called "gas" and is made up, more than anything, of loosely bound particles , expanded and with a very slight attractive force, which prevents them from having a defined shape and volume.
The release of a gas , in fact, causes it to expand freely until it fills the container in which it is contained.
Gases have a very low density , since their particles have a relative disorder: they move very quickly.
They also have a low response to the action of gravity , almost zero cohesion and variable volume , but a very high compression capacity.
Examples of gases are ozone , natural gas from cooking, helium , and the gases that make up the atmosphere .
Plasma status
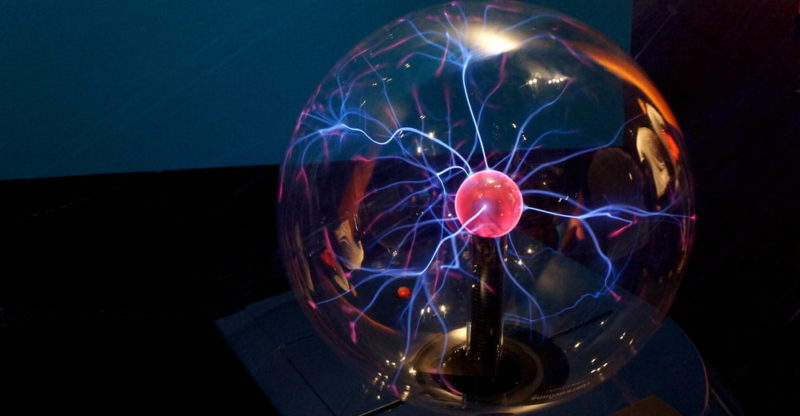
When we speak of a plasma or a substance in a plasmatic state, we speak of an ionized gas , that is, whose atoms have lost their electroneutrality and form anions (-) or cations (+).
This means that the plasmatic state is similar to gas, but with properties quite different from those of "cold gas" , such as its tendency to conduct electricity or its great response to magnetic fields .
There are two types of plasma:
- The cold plasma . That it does not cause burns because its particles do not move as fast as its electrons do.
- The hot plasma . In which atoms are bumping into each other as they move (and losing electrons) and generating light and heat in the process.
Solid State Changes
 The solid state of aggregation can become liquid or gas, through processes known as:
The solid state of aggregation can become liquid or gas, through processes known as:
- Fusion . Passage from solid to liquid. In general, it requires an increase in temperature, which induces the almost resting particles to move and expand the distance between them, relaxing the bond and, therefore, also the joint structure. An example of this process is the heating of metals in steel mills so that they can be shaped and then allowed to return to solidity (on cooling).
- Sublimation . Passage from solid to gaseous (without going through the liquid). In general, specific pressure conditions are required for this, such as those reached by snow or ice on mountain peaks, where it will never reach a melting temperature, but passes directly into steam. We can see this effect in dry ice ( frozen CO 2 ).
Changes of the liquid state
The liquid state of aggregation can become solid or gaseous through processes known as:
- Solidification . Change from liquid to solid when pressure is applied to the liquid. As a consequence, the loss of kinetic energy (heat) occurs, causing the particles to begin to move much more slowly until they compose a fixed (geometric in the case of crystallization) and constant structure. An example of this is the solidification of water.
- Freezing . Change from liquid to solid when a decrease in temperature is applied below the freezing point of the liquid.
- Vaporization . Passage from liquid to gas, generally due to the increase in temperature of the liquid, which causes the separation of its already loose bonds between particles and, therefore, the loss of its cohesion. This is what happens when we boil water.
Changes of the gaseous state
 The gaseous state can become solid, liquid or plasmatic, according to the following processes:
The gaseous state can become solid, liquid or plasmatic, according to the following processes:
- Condensation and liquefaction . These two processes involve the passage from gaseous to liquid state. Condensation occurs by loss of kinetic energy (cooling), as occurs in clouds high in the atmosphere, during the water cycle . Liquefaction occurs due to an increase in pressure that forces the particles to come together and relate again.
- Reverse deposition or sublimation . This is the name given to the change from gaseous to solid state, which can also be called crystallization. It occurs, for example, in frozen air , whose water vapor passes directly into ice crystals.
- Ionization . It occurs due to the loss of electrons from the atoms of a gas, due to an increase in the movement of its particles, which leads to the state of plasmatic aggregation.
Changes in plasma status
Matter in the plasmatic state can return to the gaseous state through a process called deionization, in which heat is removed and its particles recover the lost electrons , becoming a gas again.
Other states of aggregation
Numerous different states of aggregation are currently being experimented with, giving rise to particular substances such as ferrofluids , aerographenes and a whole range of new materials .The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring to reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
MA student of the TransAtlantic Masters program at UNC-Chapel Hill. Political Science with a focus on European Studies. Expressed ideas are open to revision. He not only covers Technical articles but also has skills in the fields of SEO, graphics, web development and coding. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

