We explain what intermolecular forces are and how they are classified. Also, what are its general characteristics and some examples?
What are intermolecular forces?
Intermolecular forces are the set of attractive and repulsive forces that occur between the molecules of matter, generally due to the presence and distribution of their electrons (polarity).
These forces occur in nature and are part of the elements and processes involved in the bond between atoms and molecules to reach more complex structures, which can occur through various types of processes, which in turn involve different types of forces.
Thus, intermolecular forces occur between molecules of diverse nature, determining many of the physical properties of the resulting substance, such as its state of aggregation, melting and boiling points, solubility, density, etc.
Importance of intermolecular forces
Intermolecular forces are fundamental forces for the construction of complex molecular structures , such as those necessary for life or to form inorganic substances of various kinds.
In addition, many physical properties of the resulting substance depend on intermolecular forces as they determine how much the minimum particles of a substance attract each other.
What is a chemical bond?

A chemical bond is a process in which two or more atoms come together to form a chemical compound. This makes the resulting structure more stable than the atoms alone. There are two classifications of chemical bonds:
- Covalent bonds. They are chemical bonds in which two atoms share electrons from their outer shell to form a stable compound.
- Not covalent. Considered “weak interactions” or lower energy, they hold molecules, ions, and parts of molecules together. These bonds occur by various types of forces: hydrogen bonds, Van der Waals forces, or London scattering forces.
Types of intermolecular forces
Ion-Ion forces
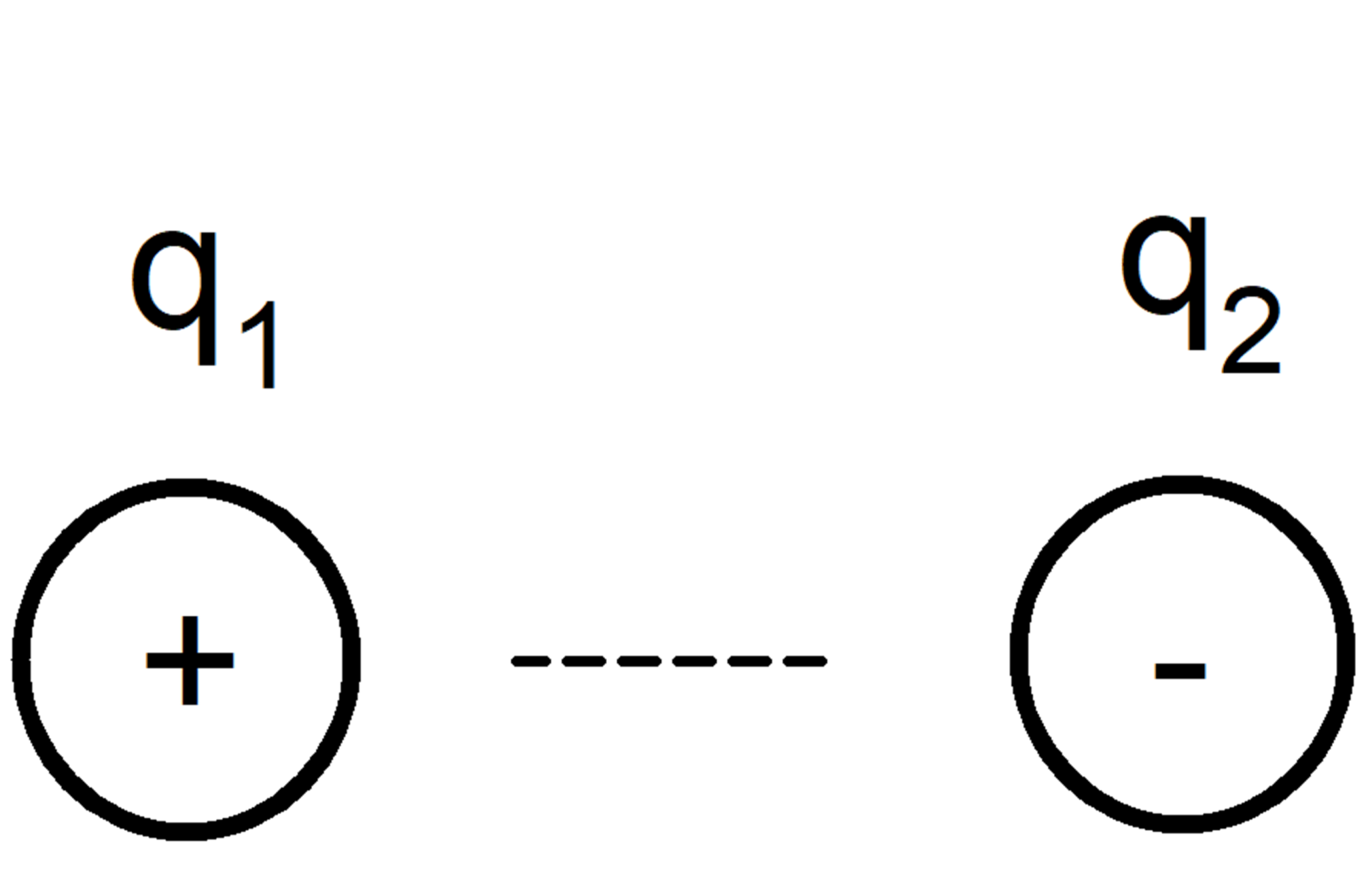
They occur between electrically charged molecules or atoms (cation if the charge is positive, anion if it is negative), according to the fundamental rule that ions with equal charges repel and with different charges attract.
Ion-dipole forces

They are attractive forces between an ion (a charged atom) and a polar molecule . The dipole (generated in the polar molecule) joins the ion through its end with an opposite charge to that of the ion, with an intensity proportional to the charge of the ion.
Many dipoles can be put together in this way, if the ion is large enough. These are the forces involved in the dissolution of salts , for example.
Induced ion-dipole forces
Very similar to the case of ion and polar molecule, but instead of the latter, it is an apolar molecule in which a dipole is induced by the electrostatic field of the ion , acquiring instantaneous polarity, but of low intensity.
Hydrophobic forces
This is the name given to the repulsive forces that certain molecules present to water , as is the case with many lipids.
These molecules, in their effort to minimize the surface that interacts with an aqueous medium, organize themselves into supramolecular aggregates.
These types of molecular constructions are fundamental for life since in many cases they allow the construction of biological membranes.
Hydrogen bonds
Hydrogen bonding occurs when a hydrogen atom in a chemical compound bonds with another strongly electronegative , such as nitrogen , oxygen or fluorine.
This leaves hydrogen with a slight partial electropositive charge, enough to interact again with other electronegative atoms (the same N, O and F) of another molecule, bridging or bonding between the two.
These bonds will be stronger the more electronegative the hydrogen-bonded atom is .
Van der Waals forces
It is known by this name to certain attractive and / or repulsive forces between molecules that do not have to do with intramolecular bonds (covalent, ionic, metallic) but with their electromagnetic or polar nature in very specific cases. Van der Waals forces are:
- Dipole-dipole forces . These are forces between two permanent dipoles, known as Keesom forces. These interactions occur between polar molecules, which have a positive pole (? + charge density ) and a negative pole ( ? - charge density ), so the positive pole interacts with the negative pole. In order to break them, it is necessary to introduce more energy than would be necessary to separate nonpolar molecules. Hydrogen bonds are often considered an example of this.
- Induced dipole-dipole forces . They are forces that are generated when a dipole induces a dipole in an apolar molecule, so they are interactions that occur between polar and apolar molecules. Then this induced dipole can interact with another dipole, as if it were Keesom forces. For this reason, for example non-polar gases (such as Cl) can be dissolved in polar solvents.
This will then allow them to come together, through their electrically opposite ends , with a force proportional to the amount of electrons they present and which have been, therefore, distributed in another way.
Examples of Compounds That Have Molecular Forces
- Cell membranes. The double layer of lipids that surrounds our cells works on the basis of hydrophobic forces, which order all lipids in the same way, with their hydrophilic heads facing outwards and their hydrophobic bodies towards the inside of the cell.
- DNA strands. The DNA double helix is held together through hydrogen bonds that give it its customary fixed structure.
- Enzymes. They react to their substrates, attracted by an ion-ion force that guarantees a strong and permanent attraction.
- Dissolution of salts. For example, sodium chloride (NaCl), has electric charges of its ions (Na + and Cl - ) that interact with the densities of electric charges of the water dipole, forming ion-dipole interactions.
Veronica is a culture reporter at Collaborative Research Group, where she writes about food, fitness, weird stuff on the internet, and, well, just about anything else. She has also covered technology news and has a penchant for smartphone stories. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

