We explain what the gaseous state is and the physical and chemical properties it presents. Also, its characteristics and examples.
What is the gaseous state?
The gaseous state is one of the states of aggregation of matter , along with the solid , liquid and plasma states. The material in the gaseous state is called "gas". It is characterized by being composed of its particles very loosely united with each other.
The particles of substances in the gaseous state have very little attractive force for each other , which is why they expand throughout the container in which they are found and adapt to their shape. This is because they vibrate with much higher energy and speed than in liquids or solids.
Due to this almost zero cohesion (forces that unite the particles) that gas particles have, they have an enormous capacity to be compressed. Compression is a process that is industrially used to liquefy gases, in this way they occupy a smaller volume and it becomes easier to transport them.
Origin of the name gas
The term "gas" was coined by the Flemish scientist Jan Baptista van Helmont , in the 17th century.
It comes from the Latin term chaos ("chaos") , since the observable state of the particles of a gas tends to dispersion and an apparent disorder.
Compared to solids and liquids, gaseous is the most chaotic state of matter .
Difference between gas and steam
Vapor is a gas that, when compressed enough at constant temperature, turns into a liquid. Gas, on the other hand, cannot transform into a liquid under these conditions.
Physical properties of gases

Gases have the following physical properties:
- They do not have a defined shape, so they take the shape of the container in which they are contained.
- They do not have a defined volume, so they tend to occupy the entire volume of the space where they are.
- They are highly compressible, that is, given the enormous space between their particles, they can be forced to occupy a smaller volume.
- Gases are fluids , like liquids, and can move with little friction from one container to another.
- The gas particles are so far apart that their total weight is less and they are less affected by gravity , so they can remain in suspension in the atmosphere .
- Gases can be more or less dense than air (depending on their nature), that is, they can rise or fall once they are released into the atmosphere.
- The taste, smell and color of gases depend on the chemical elements that make them up.
- Gases diffuse rapidly in a vacuum or between other gases.
Chemical properties of gases
The atoms and molecules of a gas are far apart and moving at very high energy levels. Therefore, it is impossible for them to remain together and rigid as in the case of solids.
The state of aggregation of matter does not alter the chemical properties of the substances that compose it. Therefore, the chemical nature of gases can vary enormously: some can be inert, others flammable, corrosive or toxic , depending on the chemical reactivity of their elements.
General gas law
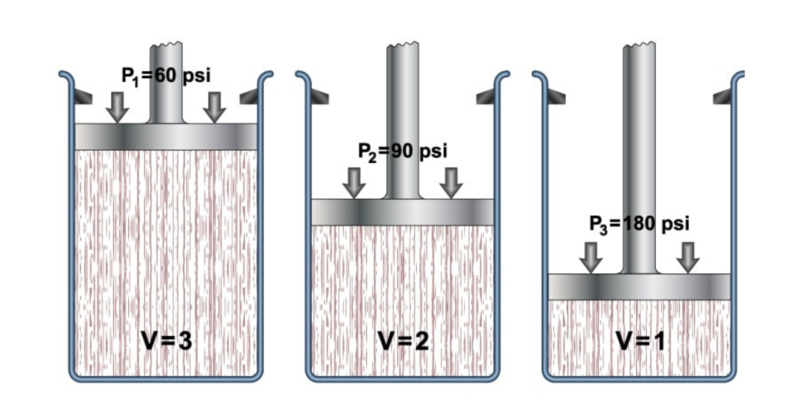
The general gas law describes the general behavior of gases , combining a set of more specific laws such as Boyle-Mariotte's Law, Charles's Law, and Gay-Lussac's Law. All of them refer to the behavior of pressure, volume and temperature of gases.
- Boyle-Mariotte law . Also called Boyle's law, formulated by Robert Boyle and Edme Marotte, it relates the pressure and volume of a gas to its temperature, so that if the temperature is constant, the other properties can be determined. This is expressed as:
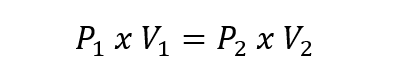
- Charles Law . This law proposes that at a given pressure, the volume occupied by a constant quantity of gas is directly proportional to its temperature expressed in kelvin. This is expressed like this:
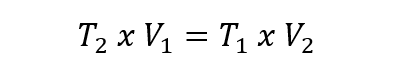
- Gay-Lussac Law . This law states that given a given and constant quantity of gas, its pressure will be directly proportional to its temperature expressed in kelvin, provided that the volume remains constant. This is expressed like this:
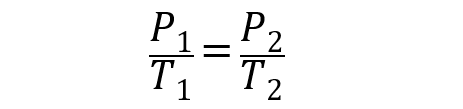
The General Gas Law proposes that, combining the previous laws, it is necessary to:
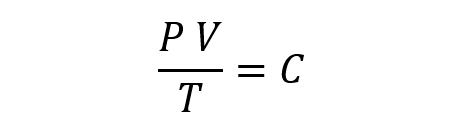
Here, P is the pressure, V is the volume, T is the temperature, and C is a constant.
Ideal gases
Ideal gases are hypothetical or theoretical gases that are a model of gases created by humans to study and explain the behavior of gases in a simpler way. To study this type of gas, the ideal gas equation of state can be used, which is represented as: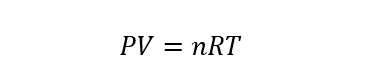
Where P is pressure, V is volume, T is temperature, n is the number of moles of gas (which must remain constant) and R is the ideal gas constant (equal to 8.314472 J / molK).
The properties of ideal gases are:
- They are made up of a certain number of molecules .
- No forces of attraction or repulsion between molecules.
- There is no collapse between the molecules, no changes in their physical nature (ie, phase changes).
- An ideal gas always occupies the same volume, under the same pressure and temperature conditions .
Real gases
Real gases are those that exist in real life. The behavior of these gases cannot be studied using the ideal gas equation of state, but its study requires the use of more complex equations . In real gases, unlike the ideal ones, we must take into account the interactions between their particles. Furthermore, phase transitions can exist in these gases.
Changes of state in gases

- Evaporation . Also called "vaporization" is the process in which a liquid goes into a gaseous state. This process occurs on a daily basis, when the heat energy of liquids increases, for example, by the action of sunlight or when they are heated. Evaporation occurs gradually and it is not necessary for all the liquid to heat up to its boiling point (the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid), it is enough to heat it up. let it evaporate little by little.
- Boil . It is the process by which, by increasing the temperature of a liquid above its boiling point, it is transformed into a gas. For this to occur, the entire mass of the liquid must be heated to a temperature equal to or greater than the boiling point.
- Sublimation . It is the process of phase change that takes from the solid state to the gaseous state, without first passing through the liquid. Although under certain conditions of pressure and temperature it can occur with ice, it occurs more frequently with other substances, such as iodine, which at 184 ºC goes directly from a solid to a gaseous state.
- Reverse sublimation . Also called deposition, this phase change is contrary to sublimation, that is, it involves the passage from gaseous directly to solid, without first passing through the liquid state. It takes place under very specific pressure conditions, which force the gas particles to join together to form rigid molecular structures. A common example of reverse sublimation occurs at the Earth's poles , on mountain tops, or in any other environment where the temperature is so low that liquid water is not formed from moisture, but ice and snow.
- Condensing . It is the opposite process to evaporation, it involves the subtraction of heat energy from a gas. As a consequence, its particles move more slowly and come together more easily, turning into liquid droplets on surfaces or falling to the ground . This is what happens in the lower atmosphere when, after moving away from the Earth's surface , the evaporated water vapor cools and forms clouds, from which the drops of water fall back to the ground: that is rain . It can also occur when ambient moisture (gaseous state) comes into contact with a cold surface, such as a bottle.
Examples of compounds in the gaseous state

Some examples of matter in a gaseous state are:
- Water vapor . When it evaporates, the water changes to the gaseous state in the form of vapor: something perfectly evident when we boil water and a column of whitish vapor emerges from the pot.
- Air . The air we breathe is a homogeneous mass of gas, a mixture of very different elements such as oxygen (O 2 ), hydrogen (H 2 ) and nitrogen (N 2 ), which are generally transparent, colorless and odorless.
- Butane . It is a gas of an organic nature and derived from petroleum , composed of flammable hydrocarbons . It is commonly used as fuel to generate fire in our kitchens, when we open the tap and this gas emerges.
- Methane . It is another hydrocarbon gas, a frequent by-product of the decomposition processes of organic matter. It can be found in swamps, sewers or even in the intestines of animals , where there are anaerobic bacteria that produce it. It has a very characteristic unpleasant odor.
Luke is passionate about fostering student involvement and connection. He studied psychology for his major and likes learning about the past. Luke aims to specialize in artificial intelligence and cybersecurity. .
Leave a reply
Your email address will not be published. Required fields are marked *Recent post

Sport: What Is It, Types, Risks, Features, Characteristics and Examples

Dogs: Emergence, Features, Characteristics, Feeding and Breeds

Story: Definition, Elements, Structure, Features and Characteristics

